




 Lung
Lung Breast
Breast Gastric
Gastric Gynecological
Gynecological Hematologic
Hematologic.jpg)


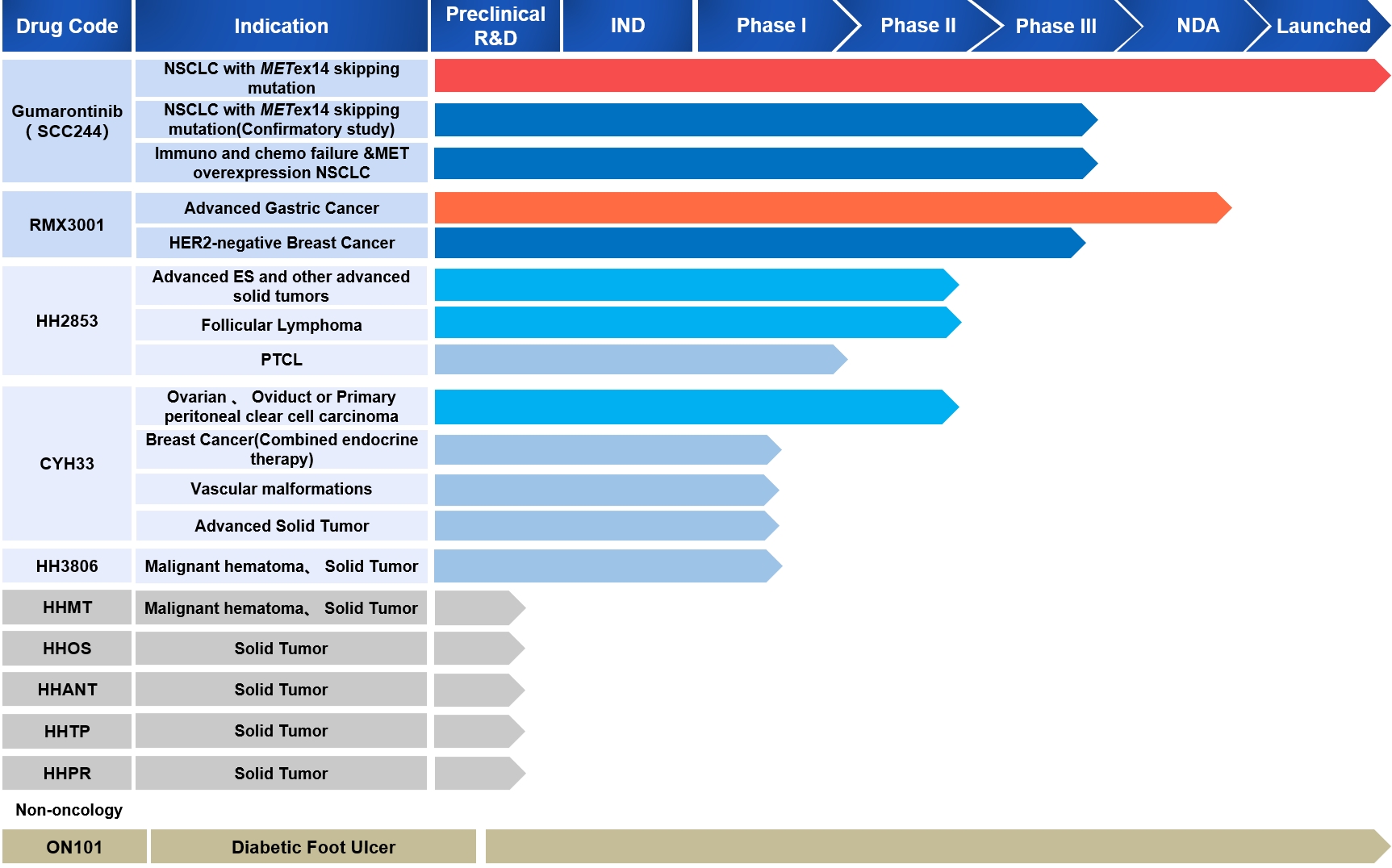
| Indication |
NSCLC with METex14 skipping mutation
NSCLC with METex14 skipping mutation(Confirmatory study)
Immuno and chemo failure &MET overexpression NSCLC
|
| Indication |
Advanced Gastric Cancer
HER2-negative Breast Cancer
|
| Indication |
Advanced ES and other advanced solid tumors
Follicular Lymphoma
PTCL
|
| Indication |
Ovarian , Oviduct or Primary peritoneal clear cell carcinoma
Breast Cancer(Combined endocrine therapy)
Vascular malformations
Advanced Solid Tumor
|
| Indication |
Malignant hematoma, Solid Tumor
|
| Indication |
Malignant hematoma, Solid Tumor
|
| Indication |
Solid Tumor
|
| Indication |
Solid Tumor
|
| Indication |
Solid Tumor
|
| Indication |
Solid Tumor
|
| Indication |
Diabetic Foot Ulcer
|